Check out our blog
Stay up-to-date with the latest developments in GDM care.
Featured
•
February 9, 2026
Updated by: Christina Inteso, PharmD, MPH, BCACP, CDCES
Since the original blog was written, the application of continuous glucose monitoring and automated insulin delivery technologies for pregnant women with type 1, type 2, or gestational diabetes: an international consensus statement1 and the 2026 American Diabetes Association Standards of Care2 were published. Updates from these references are now included.
You’re reviewing your patient’s glucose data and according to their ambulatory glucose profile (AGP), it’s all in the green. Wonderful! Green means good, right?
Not so fast. Continuous glucose monitoring (CGM) targets in pregnancy are different than non-pregnant individuals and the default target range for CGM data is set for non-pregnant patients. Targets for this population are generally 80 to130 mg/dL before a meal and less than 180 mg/dL 1 to 2 hours after the beginning of the meal. So your patient could sit between 120 to160 mg/dL all day, and be in the “green”.
However, we know that’s too high. Glucose should be lower in pregnancy—the health of the baby depends on it. We’ve had guidelines for target blood glucose ranges for fingersticks for some time. But technology is rapidly evolving and the game is changing.
If a patient has diabetes, what should their CGM targets in pregnancy be? When you look at time-in-range (TIR), what is the goal “range”? Today, we’re aiming to answer these questions and more.
What Do The Guidelines Say?
Ultimately, we want to follow evidence-based recommendations for diabetes care. A large body of research informs official recommendations, and there are well-established targets for diabetes in pregnancy.
The American Diabetes Association 2026 Standards of Care in Diabetes and American College of Obstetrics and Gynecology recommend the following glycemic targets for those with preexisting diabetes and GDM treated with insulin:2,3,
- Fasting glucose 70 to 95 mg/dL
- One-hour postprandial glucose 110 to 140 mg/dL
- Two-hour postprandial glucose 100 to 120 mg/dL
The glycemic targets for those with GDM that are not treated with insulin are:2
- Fasting glucose < 95 mg/dL
- One-hour postprandial glucose < 140 mg/dL
- Two-hour postprandial glucose < 120 mg/dL
When it comes to sensor data, one major indicator of diabetes management is TIR. TIR is the amount of time, measured in percentage, in which a person is within glucose target range. According to the ADA, the current recommendations for TIR during pregnancy (TIRp) in type 1 diabetes (T1DM) are as follows:2
- Target sensor glucose range 63–140 mg/dL: TIR, goal >70%
- Time below range (<63 mg/dL: level 1 goal <4%
- Time below range (<54 mg/dL: level 2 goal <1%
- Time above range (>140 mg/dL: goal <25%
Much more research has been done on the use of CGMs and T1DM in pregnancy than the other types of diabetes. Fortunately, CGM use during pregnancy is certainly growing more popular among patients and providers and the number of CGM studies is increasing monthly.
Fortunately, CGM use during pregnancy is certainly growing more popular among patients and providers and the number of CGM studies is increasing monthly.
Are CGM Targets in Pregnancy Different for Gestational Diabetes and Type 2 Diabetes?
While we could use the same guidelines established for T1DM, we may be accepting a higher range of glucose levels than are optimal. Researchers acknowledge we need specific targets for those with GDM and T2DM. The international consensus statement states that the most appropriate targets are still unknown, but suggest the following CGM targets in pregnancy:1,4,5
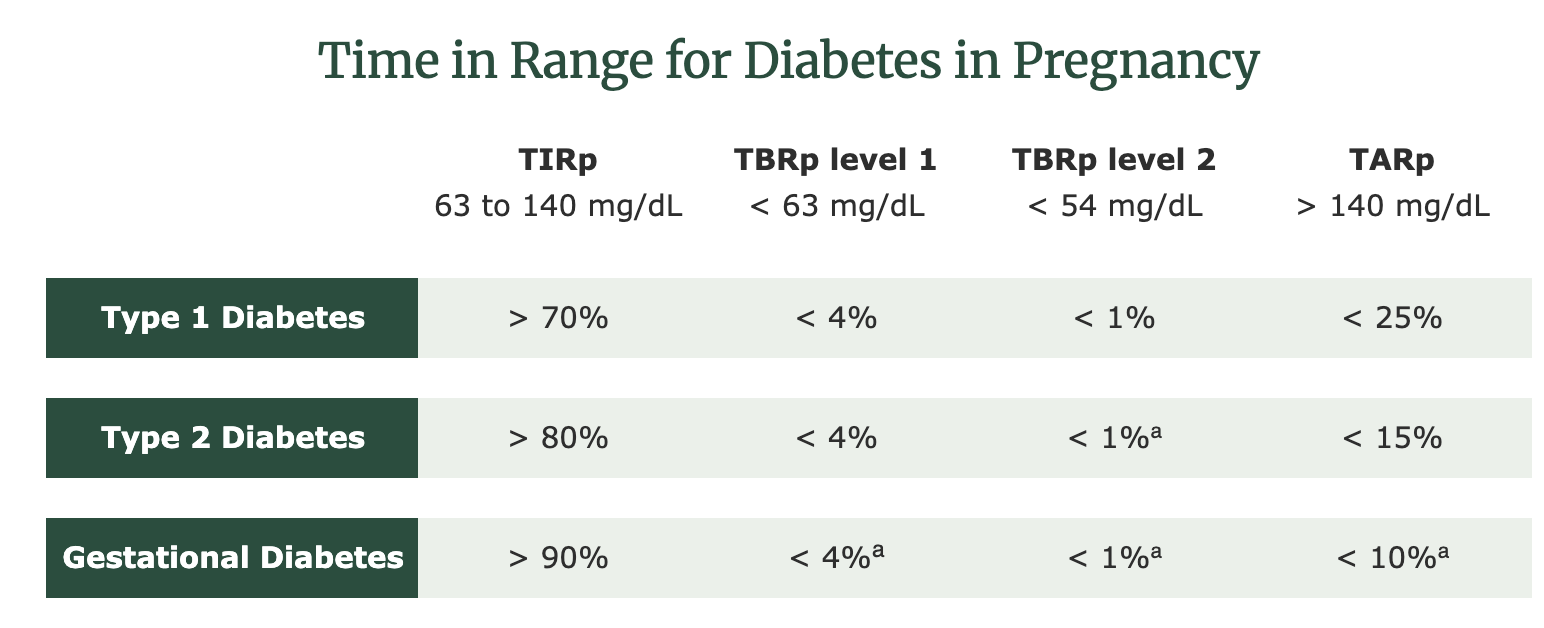
ᵃfrom Castorino K, Durnwald C, Ehrenberg S, et al. Practical Considerations for Using Continuous Glucose Monitoring in Patients with Gestational Diabetes Mellitus. J Womens Health (Larchmt). Published online October 8, 2024. doi:10.1089/jwh.2023.0864 or Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. Published 2020 Mar 16. doi:10.1186/s13098-020-00529-z
You’ll notice the TIRp is 90% for those with GDM and 80% for those with T2DM.
Why is the margin for hyperglycemia lower for those with GDM and T2DM? Simply put—it’s all about the outcomes. Hyperglycemia in pregnancy increases risk of large-for-gestational age babies, miscarriage and stillbirth, NICU admissions, neonatal hypoglycemia, and more.6 We want the evidence to inform our practice. There is plenty of data that proves CGM use can be a useful tool to manage diabetes in pregnancy.
CGM Use and Improved Glucose Levels
First, let’s start with one of the major benefits of CGM use in pregnancy—improved glucose levels. Data from the large multi-center CONCEPTT (continuous glucose monitoring in pregnant women with type 1 diabetes) trial revealed that CGM use during pregnancy was associated with improved neonatal outcomes.7
Greater time in range assumes a lower overall glucose level and reduced fetal exposure to maternal hyperglycemia. And CGM use can give patients an edge. When compared to those using fingerstick blood glucose monitoring, real-time CGM use alone can help patients with GDM achieve greater time in range.8
To determine TIR for diabetes in pregnancy, it’s helpful to first know what normal blood glucose levels are during pregnancy without diabetes.
Normal Glucose Range During Pregnancy
Given the risks of elevated glucose during pregnancy, it makes sense to aim for normal glucose ranges. With CGM data, we now know what the normal glucose range is during pregnancy.
In a multi-center study, Carlson et al evaluated pregnant participants without diabetes for an average of 123 days to determine glucose levels via Dexcom G6 CGM.9 They found mean glucose levels were between 63 to 120 mg/dL and they remained nearly stable throughout pregnancy. Values above 140 mg/dL were rare.
Whether you aim for the targets established for T1DM or you use a TIR closer to normal glucose in pregnancy levels, CGM reports and device app ranges may not provide relevant guidance. Reports and ranges are typically set for non-pregnant individuals with T1DM or T2DM. With intention, you can make diabetes technology effective for your pregnant patients.

How to Get Data Reports to Reflect Pregnancy Goals
An AGP report is more valuable when the range is accurate. By changing the range in the report settings to be 60 or 65 to 140 mg/dL (some devices will not allow you to choose 63 mg/dL, rather increments of 5 mg/dL), you’ll see when glucose levels went out of range at a quick glance.
Establishing an appropriate TIR for pregnancies complicated by gestational diabetes and T2DM will require more research. TIR cannot yet replace pre- and postprandial glucose values and targets.
Fortunately, with LilyLink, postprandial glucose logging is automated. Your patients can easily capture their glucose levels and enter meals to simplify data collection and pattern identification.
Furthermore, the LilyLink app is designed for pregnancy and your patients will see their data with those lower targets in mind. Data summaries in our EHR-integrated portal are tailored for prenatal care and simplify risk stratification, making data management clear-cut and intuitive.
.png)
Takeaways
- The preset “target” range on CGM apps and reports are not reflective of diabetes in pregnancy. A tighter range is preferred to reduce pregnancy complications related to diabetes.
- The international consensus on TIRp report states that the CGM targets in pregnancy for those with T1DM is 63 to 140 mg/dL with a goal of > 70% of time spent in range1.
- While there needs to be more research to establish concrete CGM targets in pregnancies complicated by T2DM or GDM, it’s suggested that TIR should be > 80% and > 90%, respectively, which is 15 to 20% higher than for those with T1DM1.
- Literature indicates that CGM use during pregnancy can help improve glucose levels, thereby improving outcomes.
- A review of CGM data from pregnant patients without diabetes has revealed normal glucose ranges during pregnancy—63 to 120 mg/dL, rarely exceeding 140 mg/dL.9 These values can help inform the medical community when establishing guidelines for CGM targets in pregnancy.
- LilyLink has designed their technology to serve the unique needs of diabetes care in pregnancy. Automated post-prandial logging and data reports reflective of the lower CGM targets in pregnancy reduce the complexity of diabetes management for OB practices.
Frequently Asked Questions
Is the TIR different for Type 1 Diabetes, Gestational Diabetes, and Type 2 Diabetes?
Because the body of data for TIR for T1DM in pregnancy is comprehensive, the American Diabetes Association Standards of Care in Diabetes recommends that > 70% of time should be spent in the range of 63 to 140 mg/dL. Based on the evidence that was reviewed by the international consensus on TIRp and expert opinion, it is suggested that TIRp be higher for those with T2DM and GDM (> 80% and > 90% respectively)1.
Are CGMs accurate in pregnancy?
Yes, multiple clinical trials have proven that CGMs, both real-time CGM and intermittently scanned CGM are accurate during pregnancy.10,11
What CGM metrics are useful in pregnancy?
The metrics provided by a CGM report are quite thorough. Some key metrics to review in pregnancy include:
- Percentage of CGM Active Time: Measures the amount of time the sensor was actively working and communicating with the receiver (preferred time is >70% over 14 days)
- Average Glucose
- Time in Range in pregnancy (TIRp): Percentage of readings between 63 to 140 mg/dL (please note this is not the default range for CGM reporting)
- Time Above Range in pregnancy (TARp): Percentage of readings greater than 140 mg/dL (please note this is not the default range for CGM reporting)
- Time Below Range (TBR) in pregnancy (TBRp): Percentage of readings below 63 mg/dL (please note this is not the default range for CGM reporting)
- Coefficient of Variation (CV): CV is the percentage of glycemic variability. The goal is < 36% for T1DM and ≤ 30% for T2DM. Lower numbers suggest greater glucose stability.12
References
- Benhalima K, Durnwald C, Sweeting A, et al. Application of continuous glucose monitoring and automated insulin delivery technologies for pregnant women with type 1, type 2, or gestational diabetes: an international consensus statement. Lancet Diabetes Endocrinol. 2026;14:157-77. dDoi: 10.1016/S2213-8587(25)00335-3
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2026. Diabetes Care. 2026;49(Suppl 1):S321-S338. doi:10.2337/dc26-S015
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics & Gynecology 2018;131(2):e49-e64 doi: 10.1097/AOG.0000000000002501
- Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. Published 2020 Mar 16. doi:10.1186/s13098-020-00529-z
- Castorino K, Durnwald C, Ehrenberg S, et al. Practical Considerations for Using Continuous Glucose Monitoring in Patients with Gestational Diabetes Mellitus. J Womens Health (Larchmt). Published online October 8, 2024. doi:10.1089/jwh.2023.0864
- Buhary BM, Almohareb O, Aljohani N, et al. Glycemic control and pregnancy outcomes in patients with diabetes in pregnancy: A retrospective study. Indian J Endocrinol Metab. 2016;20(4):481-490. doi:10.4103/2230-8210.183478
- Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial [published correction appears in Lancet. 2017 Nov 25;390(10110):2346. doi: 10.1016/S0140-6736(17)32712-5]. Lancet. 2017;390(10110):2347-2359. doi:10.1016/S0140-6736(17)32400-5
- Valent AM, Huertas-Pagan C, Ward L, Rickert MC, Rincon M. 259-OR: Real-time CGM achieves higher TIR among pregnant persons with GDM. Diabetes. 2024;73(Supplement_1):259–OR. doi:10.2337/db24-259-OR.
- Carlson AL, Beck RW, Li Z, et al. Glucose levels measured with continuous glucose monitoring in uncomplicated pregnancies. BMJ Open Diabetes Res Care. 2024;12(3):e003989. Published 2024 May 10. doi:10.1136/bmjdrc-2023-003989
- Polsky S, Valent AM, Isganaitis E, et al. Performance of the Dexcom G7 Continuous Glucose Monitoring System in Pregnant Women with Diabetes. Diabetes Technol Ther. 2024;26(5):307-312. doi:10.1089/dia.2023.0516
- Hussain FN, Raymond S, Feldman KM, et al. Comparison of an Intermittently Scanned (Flash) Continuous Glucose Monitoring System to Standard Self-Monitoring of Capillary Blood Glucose in Gestational Diabetes Mellitus. Am J Perinatol. 2023;40(11):1149-1157. doi:10.1055/a-2053-7650
- Danne T, Nimri R, Battelino T, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40(12):1631-1640. doi:10.2337/dc17-1600













.jpg)
.jpg)
.jpg)